
1,2,3‐Trisubstituted Indanes by Highly Diastereoselective Palladium‐Catalyzed Oxyarylation of Indenes with Arylboronic Acids and Nitroxides - Kirchberg - 2010 - Angewandte Chemie International Edition - Wiley Online Library

Mechanism and Origin of MAD-Induced Ni/N-Heterocyclic Carbene-Catalyzed Regio- and Enantioselective C-H Cyclization of Pyridines with Alkenes. - Chem. Eur. J. - X-MOL

Recent Developments in Pd0‐Catalyzed Alkene‐Carboheterofunctionalization Reactions - Garlets - 2017 - Asian Journal of Organic Chemistry - Wiley Online Library

Sensitively fluorescent detection of H2 with resazurin hydrogenation reactions catalyzed by Pd/C nanocomposites - ScienceDirect
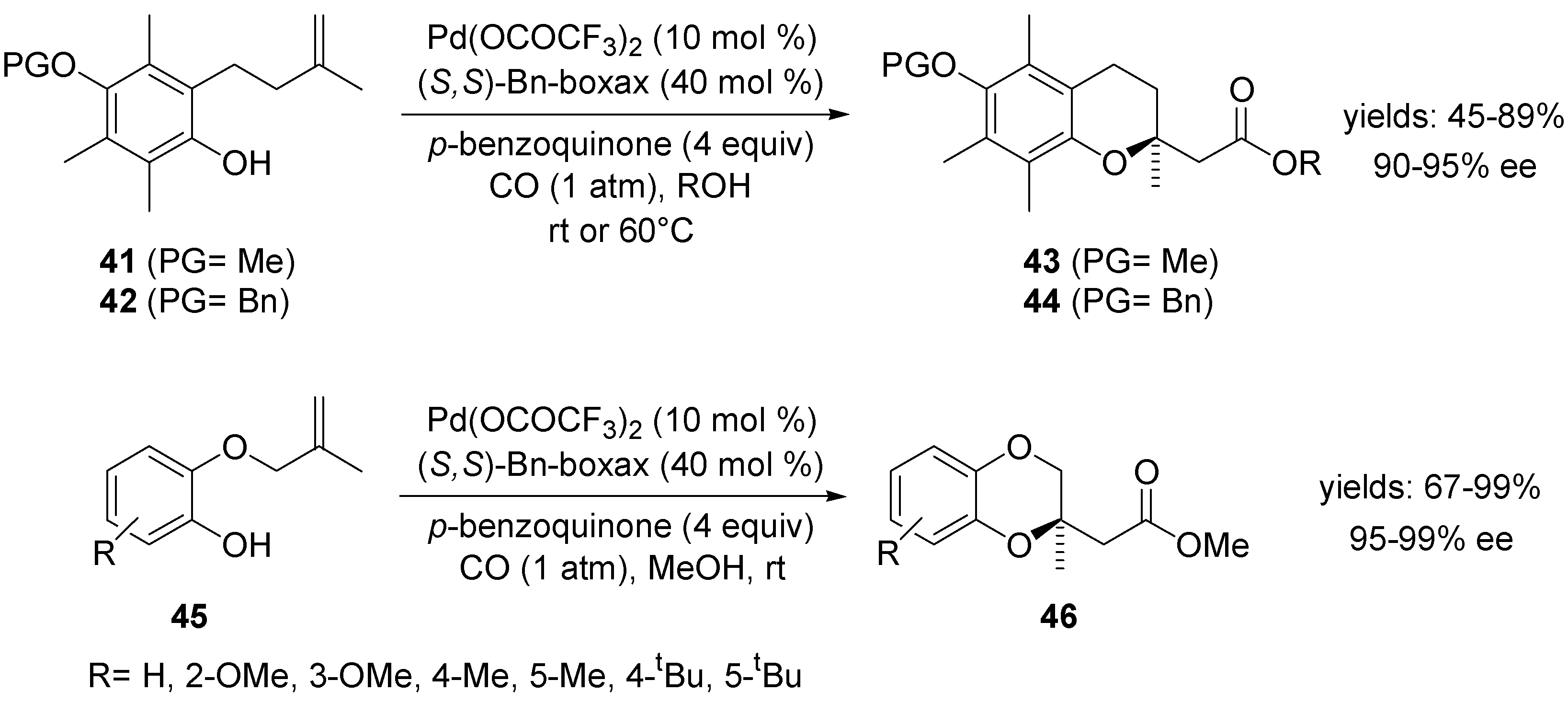
Molecules | Free Full-Text | Asymmetric Palladium-Catalysed Intramolecular Wacker-Type Cyclisations of Unsaturated Alcohols and Amino Alcohols | HTML

N-alkylation of 2-pyridone derivatives via palladium(II)-catalyzed directed alkene hydroamination - ScienceDirect

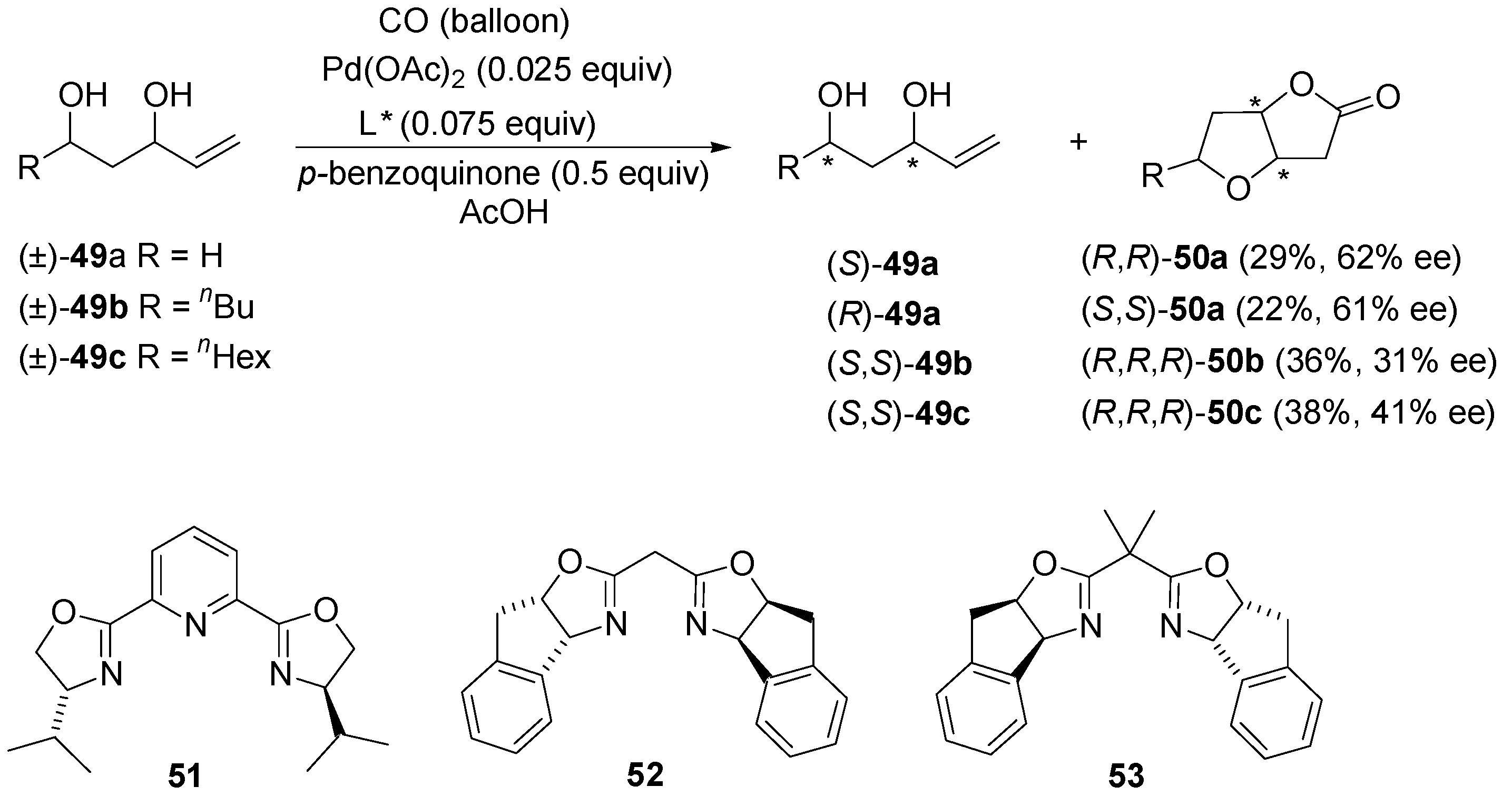
Application of Oxazoline Ligands in Palladium-Catalyzed Asymmetric Oxidative Functionalization of Alkenes

Palladium‐Catalyzed Synthesis of Benzophenanthrosilines by C−H/C−H Coupling through 1,4‐Palladium Migration/Alkene Stereoisomerization - Tsuda - - Angewandte Chemie - Wiley Online Library
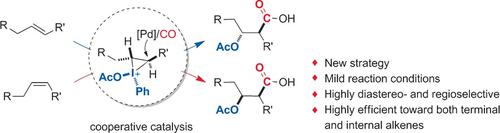
A Cooperative Strategy for the Highly Selective Intermolecular Oxycarbonylation Reaction of Alkenes using a Palladium Catalyst - Angew. Chem. Int. Ed. - X-MOL
Molecules | Free Full-Text | Asymmetric Palladium-Catalysed Intramolecular Wacker-Type Cyclisations of Unsaturated Alcohols and Amino Alcohols | HTML

Phosphaannulation of Aryl‐ and Benzylphosphonic Acids with Unactivated Alkenes via Palladium‐Catalyzed CH Activation/Oxidative Cyclization Reaction - Jeon - 2015 - Advanced Synthesis & Catalysis - Wiley Online Library
![Chemoselective Reduction catalysts | [Synthesis & Materials]Products | Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation Chemoselective Reduction catalysts | [Synthesis & Materials]Products | Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation](https://labchem-wako.fujifilm.com/us/category/images/00074-img02.png)
Chemoselective Reduction catalysts | [Synthesis & Materials]Products | Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation
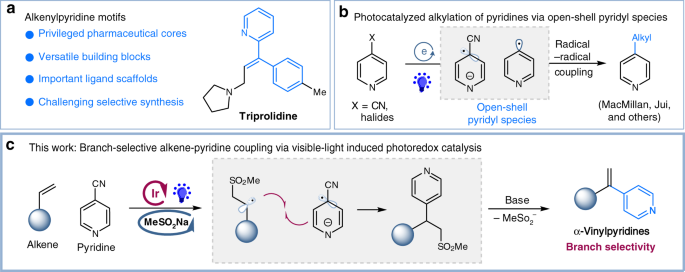
Photoredox-catalyzed branch-selective pyridylation of alkenes for the expedient synthesis of Triprolidine | Nature Communications
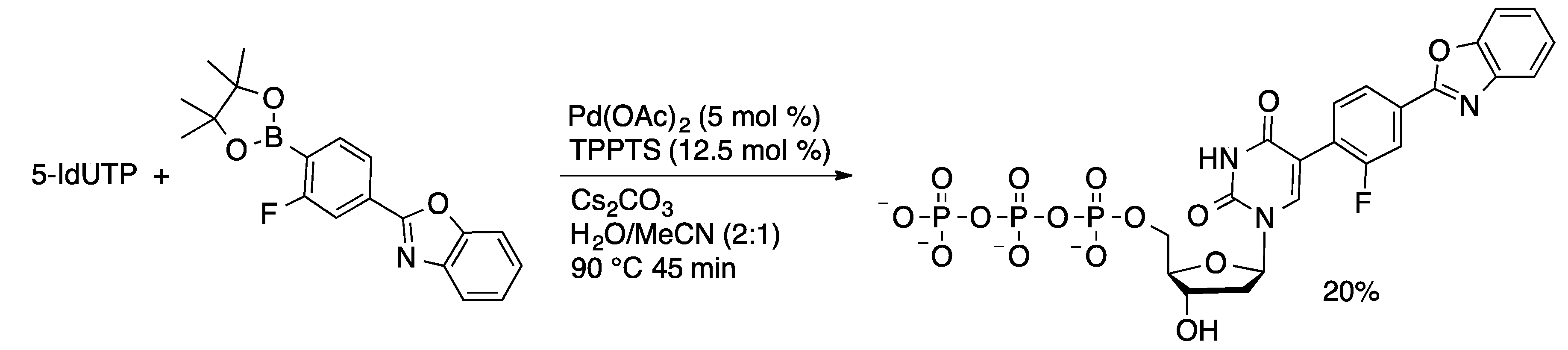
Molecules | Free Full-Text | Palladium-Catalyzed Modification of Unprotected Nucleosides, Nucleotides, and Oligonucleotides | HTML
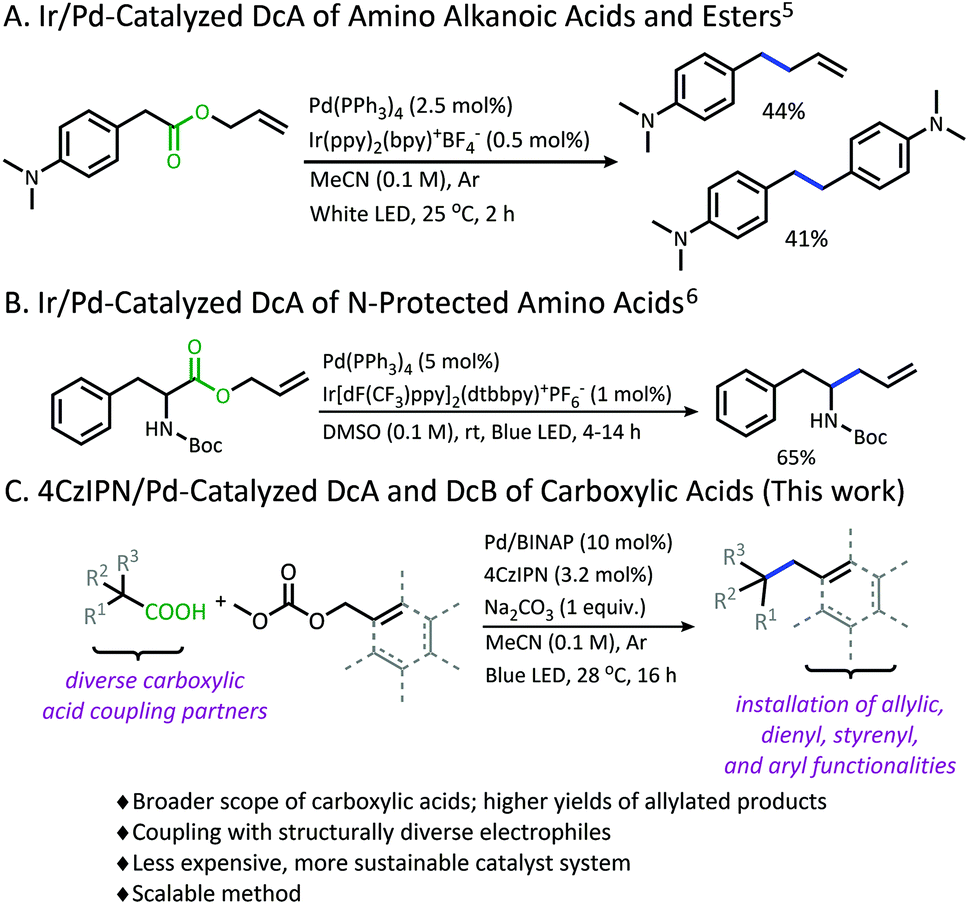
Organophotoredox/palladium dual catalytic decarboxylative Csp 3 –Csp 3 coupling of carboxylic acids and π-electrophiles - Chemical Science (RSC Publishing) DOI:10.1039/D0SC02609C

Scheme 1 Reactions used to demonstrate the catalytic farming concept.... | Download Scientific Diagram
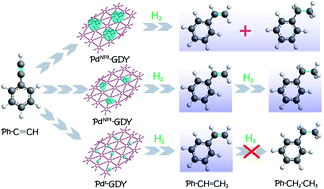
Graphdiyne-based Pd single-atom catalyst for semihydrogenation of alkynes to alkenes with high selectivity and conversion under mild conditions - Journal of Materials Chemistry A (RSC Publishing)
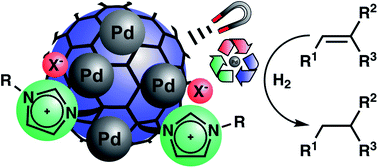
Palladium nanoparticles supported on ionic liquid modified, magnetic nanobeads – recyclable, high-capacity catalysts for alkene hydrogenation - RSC Advances (RSC Publishing)

a) The change in fluorescence intensity of compound 1 with incremental... | Download Scientific Diagram

Recent Developments in Pd0‐Catalyzed Alkene‐Carboheterofunctionalization Reactions - Garlets - 2017 - Asian Journal of Organic Chemistry - Wiley Online Library






