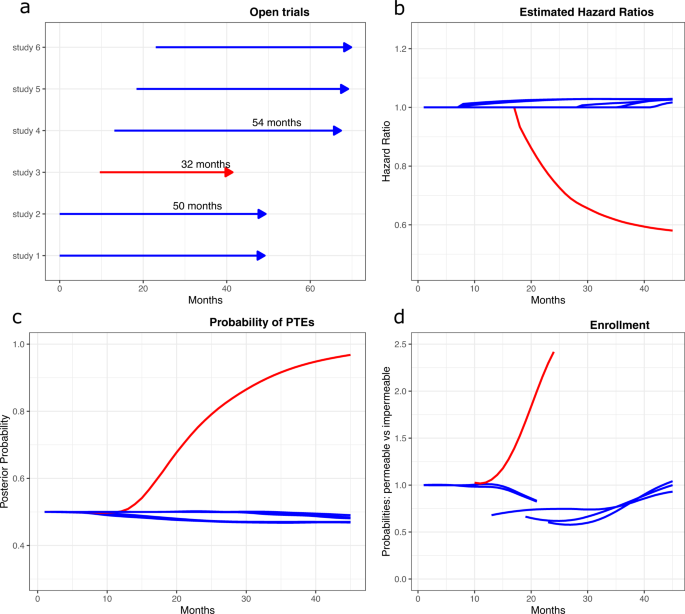
Clinical impact and quality of randomized controlled trials involving interventions evaluating artificial intelligence prediction tools: a systematic review | npj Digital Medicine
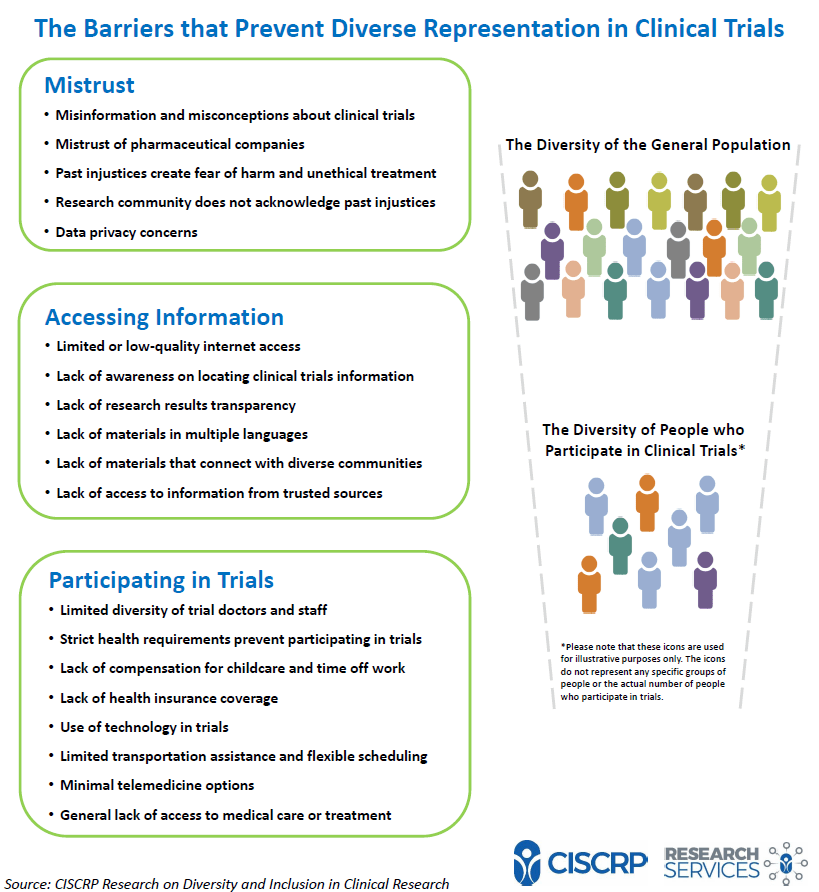
Doing Our Part: Improving Diversity in Clinical Research Participation - Center for Information & Study on Clinical Research Participation
How Frequently Do the Results from Completed US Clinical Trials Enter the Public Domain? - A Statistical Analysis of the ClinicalTrials.gov Database | PLOS ONE

A Guide to Understanding Clinical Trials: Part 2 - Five Factors to Consider When Evaluating Results - Bench Press
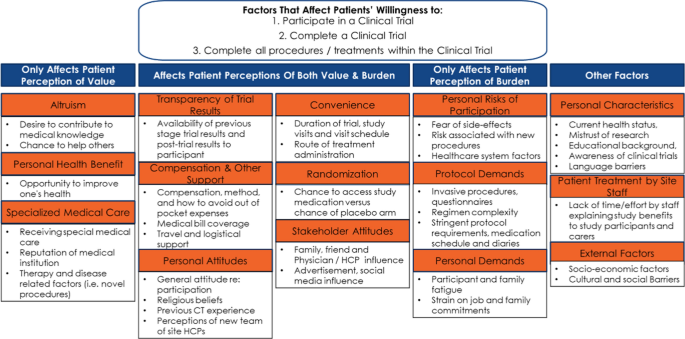
Amplifying the Voice of the Patient in Clinical Research: Development of Toolkits for Use in Designing and Conducting Patient-Centered Clinical Studies | SpringerLink
Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine
Evidence for the Selective Reporting of Analyses and Discrepancies in Clinical Trials: A Systematic Review of Cohort Studies of Clinical Trials | PLOS Medicine
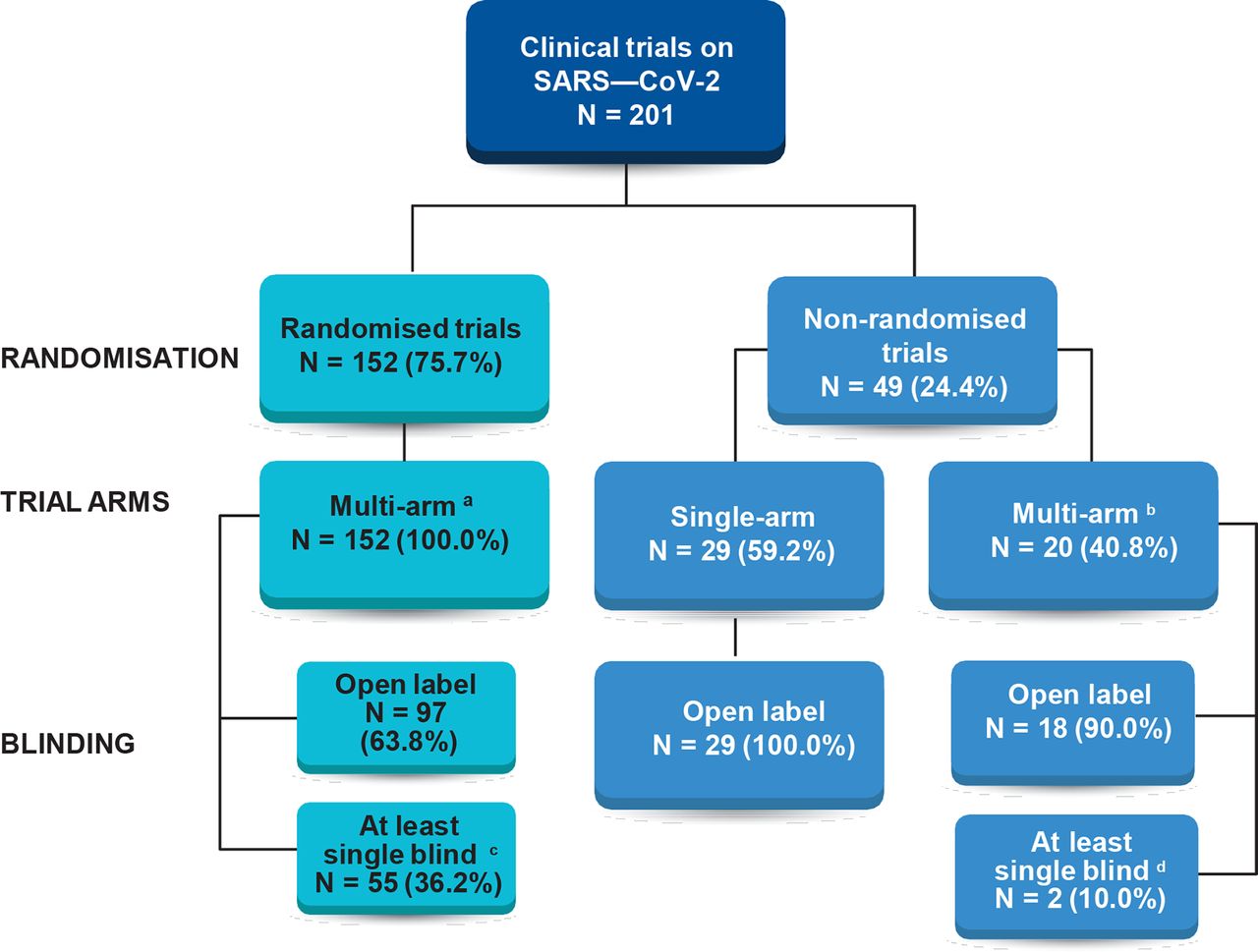
Characteristics of registered clinical trials assessing treatments for COVID-19: a cross-sectional analysis | BMJ Open

Atlanta Pediatric Research | NIH Requirements for Human Subject Research | Clinical Research Resources | Research Resources | Research | Emory + Children's + GT | Atlanta Pediatric Research Alliance
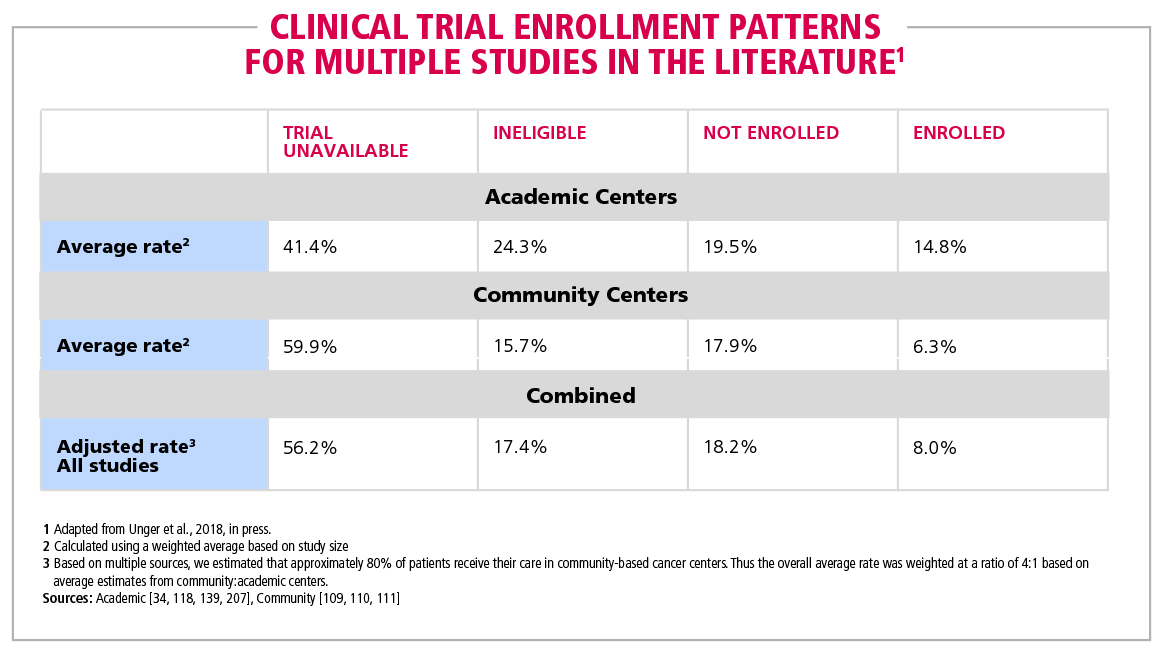
Table 1. Clinical trial enrollment patterns for multiple studies in the literature | American Cancer Society Cancer Action Network