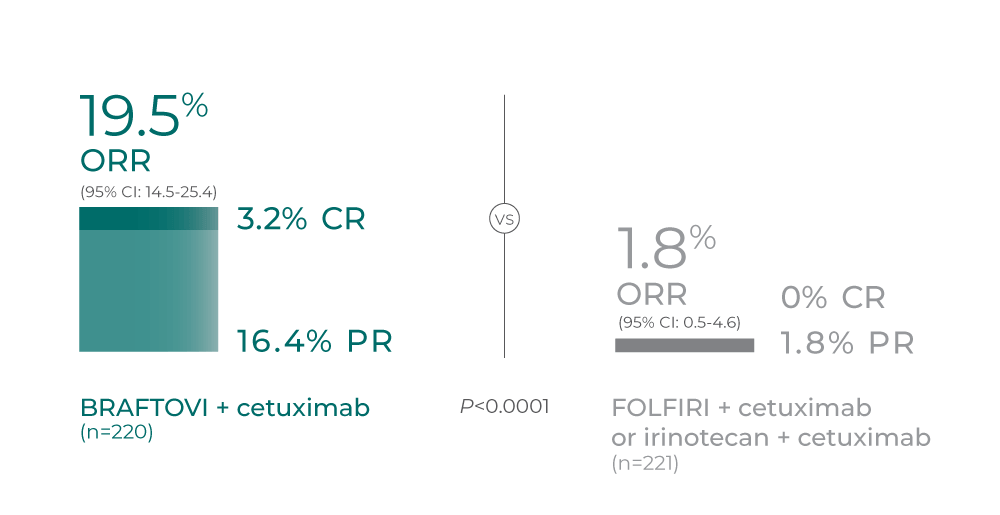
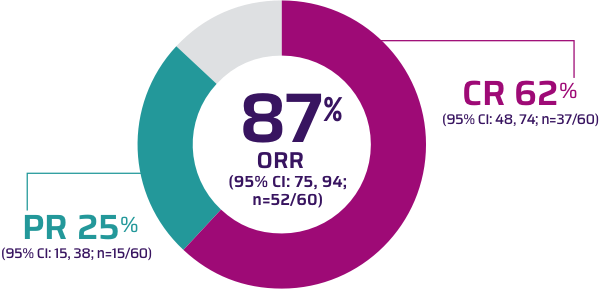
Response rates Overall response rate and depth of response according to... | Download Scientific Diagram
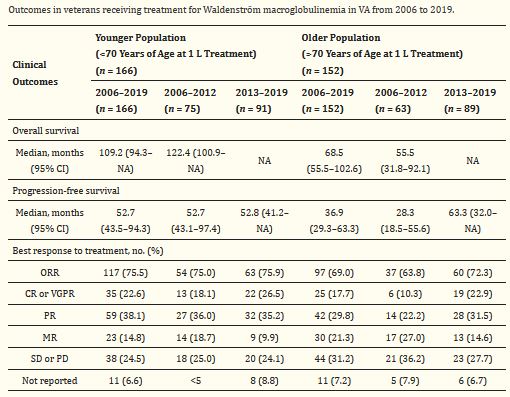
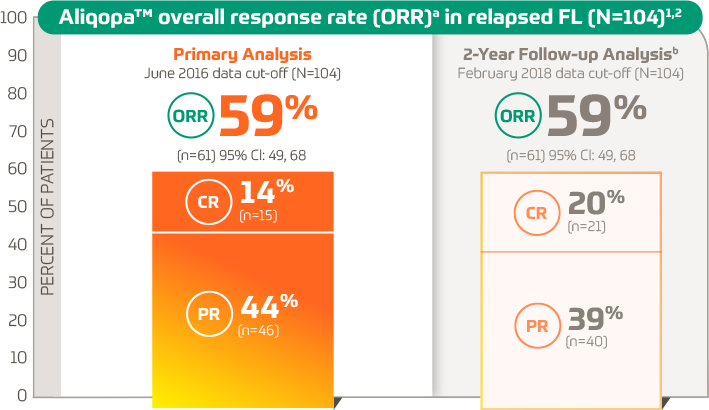
Response Rates for Waldenström Macroglobulinemia Improve With Use of New First-Line, Better-Tolerated Treatments - U.S. Medicine
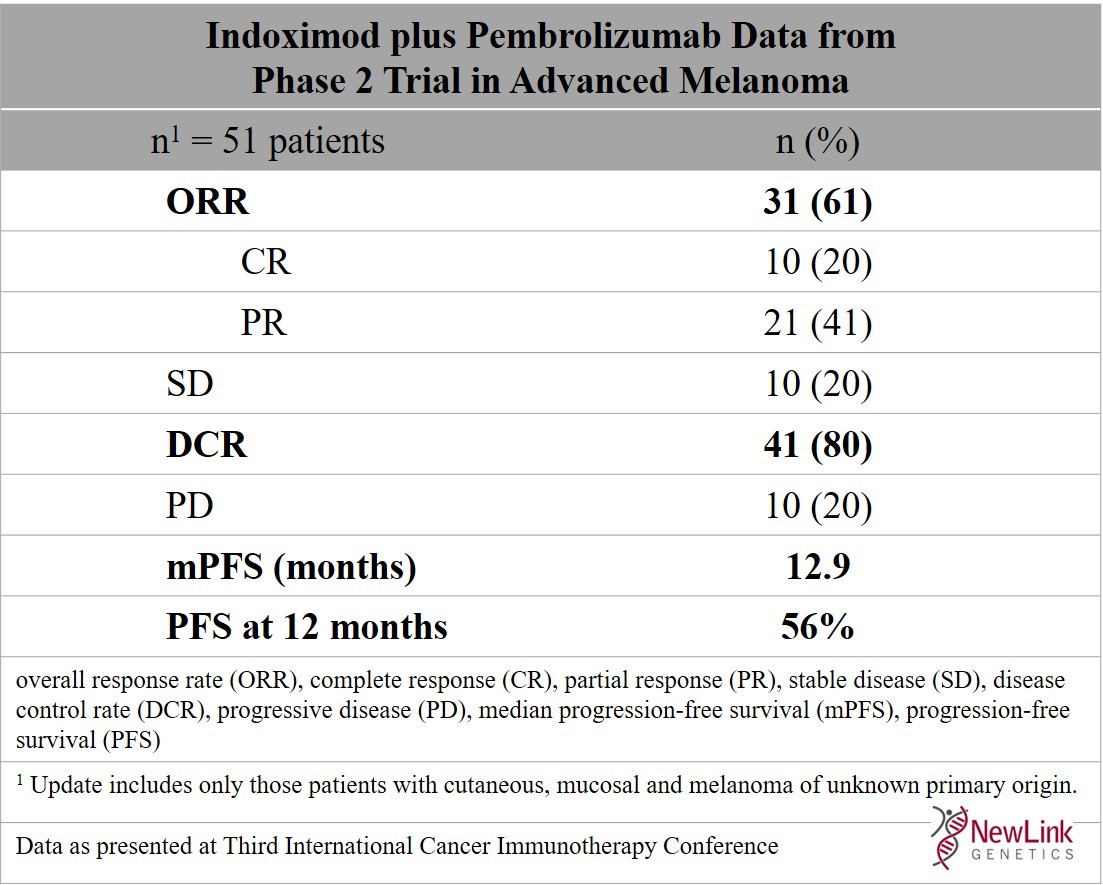
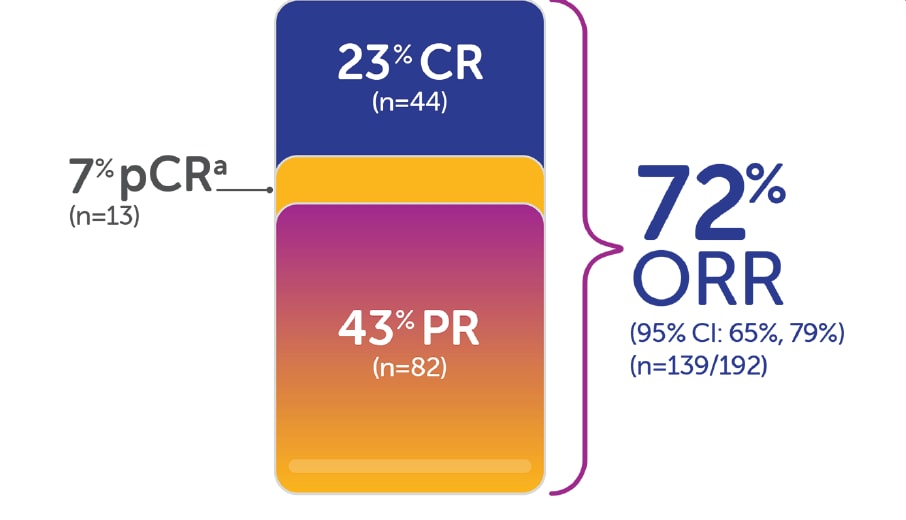
Updated Data for Indoximod Plus KEYTRUDA® (pembrolizumab) Demonstrate Improvement of Response Rate for Patients with Advanced Melanoma | Business Wire
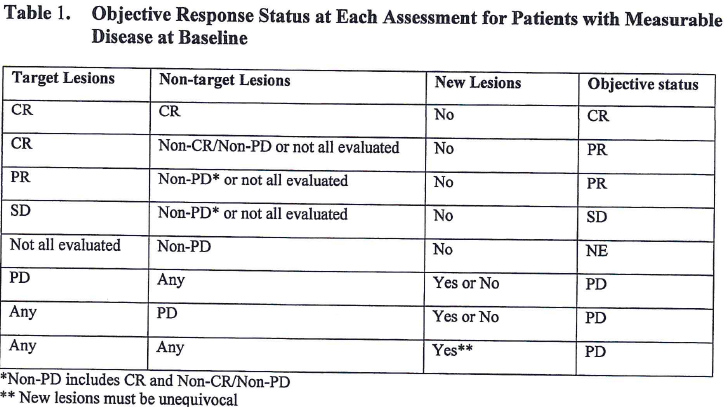
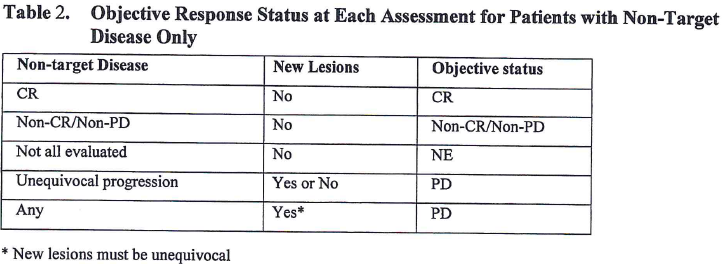
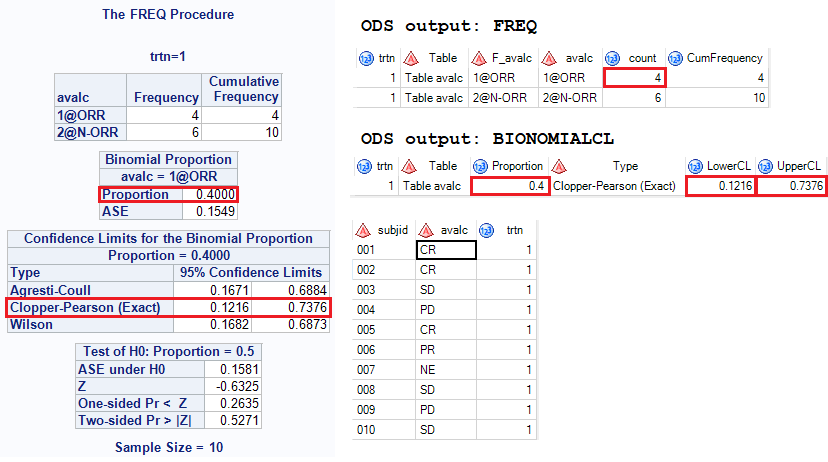
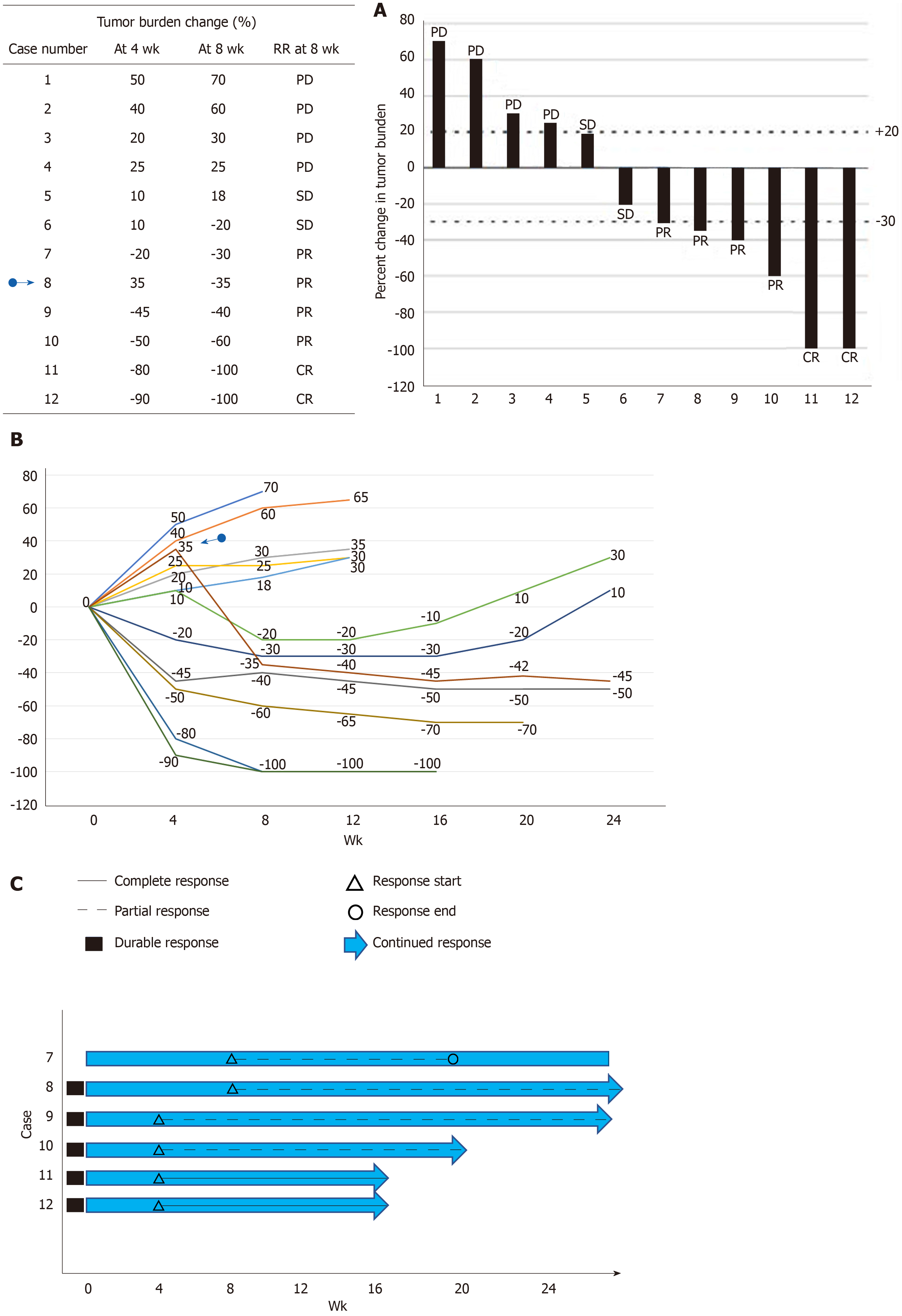
![PDF] Objective response rate assessment in oncology: Current situation and future expectations | Semantic Scholar PDF] Objective response rate assessment in oncology: Current situation and future expectations | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/31979901b045184c8053075e4956319c5588d33c/4-Table1-1.png)